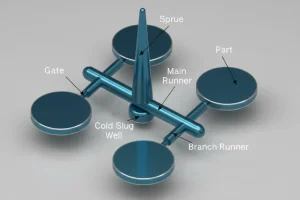
– MUD (Master Unit Die) systems reduce tooling costs by up to 66% compared to standard mold bases, essential for budget-conscious medical prototyping.
– Quick-change capabilities allow for 5-minute changeovers, significantly boosting uptime in ISO class cleanroom molding environments.
– MUD inserts facilitate rapid medical manufacturing by bridging the gap between prototype and high-volume production tools.
– Compatibility with standard injection molding machines ensures seamless integration into existing ISO 13485 compliant workflows.
What Is a Master Unit Die (MUD) System in the Context of Medical Manufacturing?
A Master Unit Die (MUD) system is a quick-change tooling framework designed to separate the mold base (the frame) from the molding surface (the insert). In medical injection molding1, the MUD frame remains clamped in the injection molding machine, while only the insert containing the cavity and core is removed and replaced.
This modular approach contrasts with standard mold bases where the entire heavy steel structure must be removed for every part change. For medical device manufacturers operating under ISO 134852 (Medical devices – Quality management systems) standards, this agility supports the rigorous demands of medical device prototyping and validation runs without the prohibitive cost of full-scale production tooling.
Core Components of a MUD System
- U-Frame or H-Frame: The standardized exterior frame that mounts to the machine platen.
- Companion Insert: The interchangeable block containing the part geometry, runner system, and ejection pins.
- Quick Disconnects: Standardized water and air fittings that allow rapid decoupling during changeovers.
MUD systems are fully compatible with ISO 13485 and FDA validation requirements when managed correctly.Правда
The tooling format does not dictate compliance; the validation of the process (IQ/OQ/PQ) and part quality determines regulatory acceptance.
MUD inserts require specialized injection molding machines to operate.Ложь
MUD frames are designed to fit standard injection molding machines; no specialized machinery is required, only the specific MUD frame adapter.

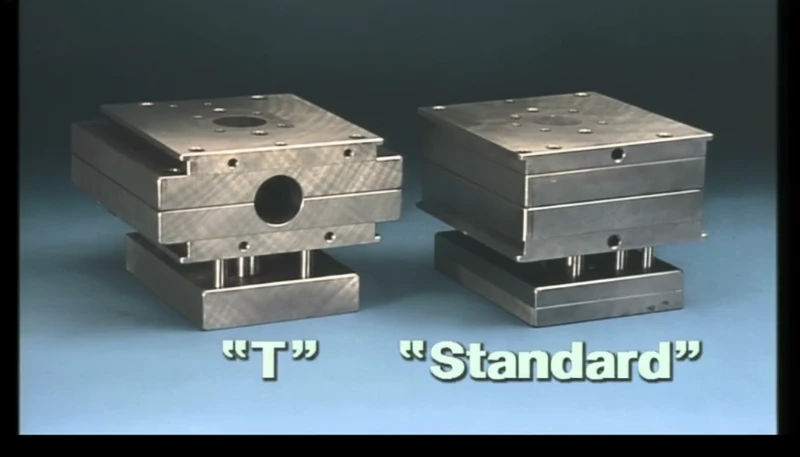
How Do MUD Systems Compare to Standard Mold Bases?
To understand the shift toward rapid medical manufacturing, one must compare the technical parameters of MUD systems against traditional standalone tooling.
| Параметр | Standard Mold Base | MUD System (Quick-Change) | Medical Impact |
|---|---|---|---|
| Стоимость оснастки | 100% (High) | 30% – 40% of Standard | Reduces financial risk during R&D and clinical trials. |
| Changeover Time | 1 – 4 Hours | 5 – 15 Minutes | Minimizes downtime in expensive cleanroom molding environments. |
| Время выполнения | 8 – 12 Weeks | 2 – 5 Weeks | Accelerates "Time to Market" for 510(k) submissions. |
| Storage Footprint | Large (Full Base) | Compact (Inserts Only) | Critical for facilities with limited controlled storage. |
| Tonnage Range | Unlimited | Typically < 500 Tons | Suitable for small to medium medical components (e.g., syringes, housings). |
| Термическая стабильность | High (Massive Steel) | Moderate (Less Mass) | Requires precise cooling design for semi-crystalline medical polymers. |

Why Is Speed and Flexibility Critical for Medical Device Prototyping?
The medical industry creates a unique pressure cooker where medical device prototyping3 must be fast, yet functionally identical to the final product for testing.
1. Accelerating the Iteration Loop
In the early stages of product development, design changes are frequent. Using ISO 13485 tooling standards within a MUD framework allows engineers to machine only the insert. If a design change is required, modifying or replacing a small insert is significantly faster and cheaper than altering a full mold base.
2. The "Bridge Tooling" Strategy
Manufacturers use MUD inserts as "bridge tools." These are used to produce valid, production-grade parts for FDA testing and market entry while the high-cavity, hardened steel production mold is being built (which can take months). This ensures no gap in supply.
3. Cleanroom Economy
Operating an ISO Class 7 or 8 cleanroom is expensive. Every minute a machine is down for a mold change is lost revenue. MUD systems allow a single operator to swap inserts in under 15 minutes without needing an overhead crane, keeping the cleanroom molding4 environment productive and reducing contamination risks associated with heavy equipment movement.
MUD inserts allow for the use of production-grade medical resins like PEEK and Polycarbonate.Правда
MUD inserts can be machined from high-grade stainless steel (e.g., 420 SS) to handle high temperatures and abrasive medical polymers.
MUD systems are inherently lower precision than standard mold bases.Ложь
Precision is determined by the machining tolerance of the insert and the machine's capability, not the frame system. MUDs successfully mold micron-tolerance medical parts.

What Are the Advantages and Disadvantages of MUD Systems?
While beneficial, MUD systems are not a universal solution. Below is a technical breakdown of pros and cons specifically for the medical sector.
| Характеристика | Преимущества | Недостатки |
|---|---|---|
| Эффективность затрат | Eliminates the cost of purchasing a mold base for every project. Clients pay only for the cavity steel. | Not cost-effective for ultra-high volume (millions of parts) where dedicated multi-cavity tools are preferred. |
| Скорость | Supports rapid medical manufacturing; faster heat-up and cool-down due to lower thermal mass. | Limited cooling channel real estate compared to large mold bases, which can extend cycle times for thick parts. |
| Validation | Simplified validation for family parts (similar geometry) sharing the same frame. | Care must be taken to ensure the "Universal Frame" is well-maintained; wear on the frame affects all inserts. |
| Material Usage | Reduced sprue and runner waste in many configurations. | Limited slide and lifter action space for highly complex undercuts. |
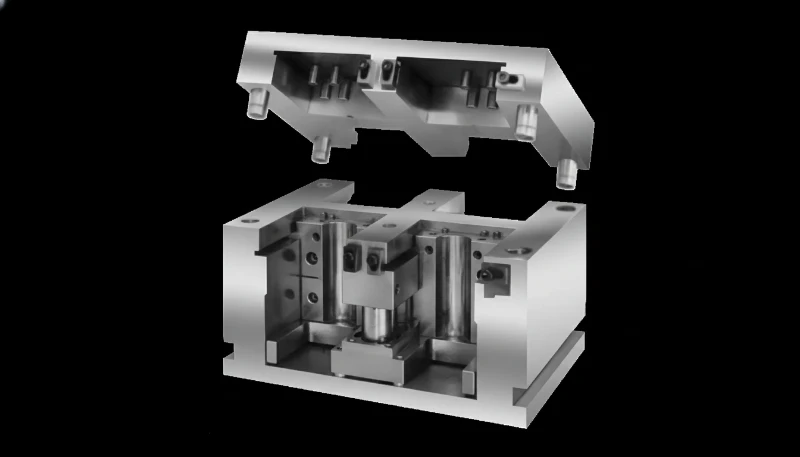
What Practical Tips Optimize MUD Systems for Medical Applications?
To ensure ISO 134852 tooling compliance and optimal performance, engineers should follow these best practices:
- Select the Right Steel: Do not use aluminum for medical MUD inserts if the parts are for clinical trials. Use pre-hardened steel (e.g., P20) or stainless steel (e.g., 420 SS) to ensure the surface finish meets sanitary requirements and resists corrosion from sterilization.
- Dedicated Water Manifolds: In cleanrooms, water leaks are disastrous. Use dripless quick-connect couplers integrated directly into the MUD insert plate to prevent water spillage during changeovers.
- Surface Treatments: For parts requiring easy release without mold spray (which is often restricted in medical molding), apply permanent coatings like Nickel-PTFE or DLC (Diamond-Like Carbon) to the insert.
- Standardize Ejection: Design inserts to utilize the standard ejector pattern of the MUD frame to avoid custom coupling requirements that slow down changeovers.

Where Are MUD Systems Applied in Medical Manufacturing?
The versatility of MUD frames makes them ideal for specific medical sectors:
- Surgical Consumables: Trocars, scalpel handles, and suture clips where volumes are moderate, and designs evolve.
- Orthopedics: Trial implants made from Polyetheretherketone (PEEK) or Polyphenylsulfone (PPSU).
- Diagnostics: Microfluidic chips and test cartridges requiring high optical clarity (using Polycarbonate or COP).
- Wearable Devices: Housings for insulin pumps or glucose monitors requiring soft-touch overmolding (TPE over rigid substrates).
What Is the Step-by-Step Process for Implementing MUDs?
- Assessment: Analyze part volume and complexity. If annual volume is <250,000 and the part fits within standard MUD insert dimensions (e.g., 8×10, 10×12), proceed.
- Frame Selection: Purchase or lease a high-quality U-Frame compatible with existing cleanroom presses.
- Insert Fabrication: CNC machine the companion insert using medical-grade steel.
- Integration: Install the frame into the injection molding machine and hook up cooling/heating lines.
- Validation (IQ/OQ/PQ): Perform Installation Qualification, Operational Qualification, and Performance Qualification on the specific insert/frame combination.
- Production: Run the batch.
- Storage: Remove the lightweight insert, clean it, and store it in a compact, controlled environment.
Часто задаваемые вопросы (FAQ)
Is a MUD system compliant with FDA regulations?
Yes. The FDA regulates the medical device and the process outcome, not the specific tooling hardware. As long as the MUD system produces parts that meet the specifications and the process is validated (IQ/OQ/PQ), it is fully compliant.
Can MUD systems handle high-temperature medical polymers?
Yes. MUD inserts can be manufactured from high-grade stainless steel capable of withstanding the high mold temperatures required for engineering resins like PEEK, PEI (Ultem), and PPSU. However, thermal isolation plates may be needed between the insert and the frame.
How does a MUD system affect cleanroom classification?
MUD systems generally improve cleanroom compliance. Their quick-change nature generates less particulate matter than standard mold changes (no crane grease, less heavy movement) and reduces the time personnel spend inside the controlled environment.
Are MUD inserts suitable for multi-shot (2K) molding?
Yes, but with limitations. Rotary platen MUD frames exist, but for complex overmolding (e.g., soft-grip surgical handles), specific rotary MUD frames or robotic transfer systems are required.
What is the lifespan of a MUD insert compared to a standard mold?
If made from the same steel grade (e.g., 420 Stainless), the cavity lifespan is identical. The limitation lies in the shared frame; if the frame wears out, flash may develop across all inserts used in that frame. Regular frame maintenance is critical.

Резюме
Medical device manufacturers are increasingly switching to Master Unit Die (MUD) systems to solve the "Iron Triangle" of manufacturing: Speed, Cost, and Quality. By decoupling the molding surface from the mold base, MUD systems enable rapid medical manufacturing and cost-effective medical device prototyping. When properly managed within an ISO 13485 environment, MUDs provide a validated, scalable path from concept to clinical trial, allowing manufacturers to bring life-saving devices to market faster and more efficiently.
-
Learn about the process of medical injection molding and its importance in producing high-quality medical devices. ↩
-
Understand the significance of ISO 13485 in ensuring quality management systems for medical devices. ↩ ↩
-
Discover effective strategies for prototyping medical devices that meet regulatory standards. ↩
-
Explore the role of cleanroom molding in maintaining product quality and compliance in medical manufacturing. ↩