Medical injection molding is a specialized manufacturing process designed to produce durable, biocompatible, and precise components for the healthcare industry. Unlike general-purpose molding, it requires adherence to strict regulatory standards such as **ISO 13485** and **FDA 21 CFR Part 820**. The process typically utilizes medical-grade resins (e.g., PEEK, PC, LSR) and operates within **ISO 14644** classified cleanrooms to minimize particulate contamination. Key priorities include process validation (IQ/OQ/PQ), full traceability, and “zero defect” quality control.
Definition
Medical Injection Molding is the process of shaping medical-grade plastic or Liquid Silicone Rubber (LSR) materials into specific geometries using a heated barrel, mixing screw, and a precision mold tool.
This process is distinguished by its environment and compliance requirements. It must ensure that the final product meets biocompatibility standards (such as ISO 10993) and maintains sterility or sterilizability. It encompasses everything from high-volume disposable consumables (syringes, test tubes) to high-precision implantable devices and surgical instruments.
Medical injection molding requires strict adherence to ISO 13485 quality management standards.True
ISO 13485 is the global standard for medical device quality management systems, ensuring consistency, risk management, and regulatory compliance.
Any clean environment is sufficient for manufacturing medical devices.False
Medical molding typically requires certified ISO Class 7 or 8 cleanrooms to control airborne particulate concentration to specific regulatory limits.

Key Technical Parameters
The following parameters define the operational window for high-quality medical molding.
| Parameter | Typical Range / Requirement | Unit / Note |
|---|---|---|
| Clamp Tonnage | 30 – 500+ | Tons (Depends on part size) |
| Cleanroom Class | Class 7 (10,000) or Class 8 (100,000) | ISO 14644 Standard |
| Injection Pressure | 100 – 200 | MPa (High pressure for thin walls) |
| Melt Temperature | 200 – 400 | °C (Material dependent; PEEK is higher) |
| Mold Steel Hardness | 48 – 56 | HRC (Stainless steel required) |
| Dimensional Tolerance | ±0.001 to ±0.05 | mm (Precision is critical) |
| Material Grade | USP Class VI / ISO 10993 | Must be Biocompatible |

Advantages and Disadvantages
| Advantages | Disadvantages |
|---|---|
| High Precision & Repeatability: Capable of producing millions of identical parts with micron-level tolerances. | High Initial Cost: Mold tooling and cleanroom setup costs are significantly higher than standard molding. |
| Regulatory Compliance: Established processes for FDA and ISO certification streamline final device approval. | Strict Validation: Requires lengthy IQ/OQ/PQ (Installation, Operational, Performance Qualification) protocols. |
| Material Versatility: Supports advanced resins like Polyetheretherketone (PEEK) and bio-absorbable polymers. | Long Lead Times: Tool design, fabrication, and validation can take 12–16 weeks before production starts. |
| Scalability: Ideally suited for high-volume production of disposables. | Design inflexibility: Once the mold is validated, design changes are costly and require re-validation. |
Common Applications
- Implantable Components: Screws, plates, and casings made from PEEK or bio-resorbable polymers.
- Surgical Instruments: Single-use handles, forceps, and trocars requiring sterilization resistance.
- Drug Delivery Systems: Syringes, insulin pens, and inhaler components utilizing medical-grade Polycarbonate1.
- Diagnostic Consumables: Microfluidic chips, petri dishes, and pipettes requiring high optical clarity.
- Catheters & Tubing Connectors: Utilizing flexible materials like Thermoplastic Elastomers (TPE) or LSR.
Stainless steel molds are preferred in medical molding to prevent corrosion and particulate contamination.True
Hardened stainless steel resists corrosion from frequent sterilization and ensures no rust particles contaminate the medical components.
Standard industrial plastics can be used for medical devices if they are sterilized.False
Only medical-grade resins that have passed ISO 10993 biocompatibility testing are safe for contact with human tissue or fluids.

Stepwise Process: Medical Device Molding Cycle
To ensure compliance and quality, medical molders follow a rigorous workflow.
-
Design for Manufacturability (DFM):
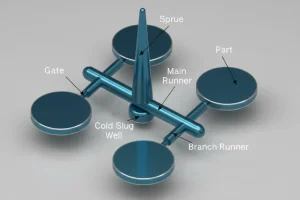
Engineers analyze the part geometry to ensure it can be molded without defects. This includes optimizing draft angles, wall thickness, and gate locations to minimize flow stress. -
Material Selection:
Choose a resin based on mechanical needs and regulatory requirements (e.g., Polypropylene (PP) for chemical resistance, Polycarbonate (PC) for impact strength). The material must have a Device Master File (DMF) with the FDA. -
Mold Tooling Fabrication:
Molds are machined from high-grade stainless steel (e.g., 420 SS) to prevent rust and facilitate easy cleaning. Surface finishes are often polished to SPI-A2 or better to prevent bacterial adhesion. -
Process Validation (IQ/OQ/PQ):
- Installation Qualification (IQ): Verifying equipment is installed correctly.
- Operational Qualification (OQ): Establishing the process window (limits for temp, pressure, time).
- Performance Qualification (PQ): Verifying process stability over a standard production run.
-
Cleanroom Production:
Molding occurs in a controlled environment (ISO Class 7 or 8). Robots typically remove parts to prevent human contamination. -
Quality Assurance & Packaging:
Parts undergo automated vision inspection. Packaging is often done within the cleanroom to maintain the bio-burden level before terminal sterilization2.

FAQ: Injection Molding for Medical Devices
Q1: What is the difference between ISO 13485 and ISO 9001?
ISO 13485 is specific to the medical device industry. While it is based on ISO 9001 (general quality management), it places extra emphasis on risk management, documentation, sterilization, and traceability, rather than just customer satisfaction and continuous improvement.
Q2: What is Liquid Silicone Rubber (LSR) molding?
Liquid Silicone Rubber (LSR) molding uses a two-component liquid system cured by heat (vulcanization) rather than cooled like thermoplastics. It is ideal for medical seals, masks, and catheters due to its biocompatibility, flexibility, and resistance to bacterial growth.
Q3: What are the cleanroom requirements for medical molding?
Most medical molding takes place in ISO Class 8 (100,000 particles/ft³) or ISO Class 7 (10,000 particles/ft³) cleanrooms. These environments control temperature, humidity, and positive pressure to prevent dust and microbes from contaminating the parts.
Q4: What is IQ/OQ/PQ validation?
This is a three-step protocol required by regulatory bodies to prove that the manufacturing process can consistently produce products meeting predetermined specifications. It validates the equipment (IQ), the process parameters (OQ), and the consistent performance under load (PQ).
Q5: Can medical injection molded parts be sterilized?
Yes, but the resin must be selected accordingly. Materials like Polysulfone (PSU) and Polyetherimide (PEI) can withstand repeated autoclaving (steam sterilization), while others may degrade and are better suited for Gamma or Ethylene Oxide (EtO) sterilization.
Validation processes like IQ/OQ/PQ are legally required for medical device manufacturing.True
Regulatory bodies like the FDA and ISO require validation to prove the manufacturing process consistently produces safe and effective products.
Medical injection molding is the best choice for low-volume prototyping.False
Due to high tooling costs, 3D printing or CNC machining is usually preferred for low-volume prototypes; molding is optimized for mass production.
Summary
Injection molding for medical devices is a discipline defined by risk management and process control. Unlike consumer goods, where aesthetics or cost may drive decisions, medical molding prioritizes patient safety through strict adherence to ISO 134853 and material biocompatibility. By leveraging high-precision electric molding machines, automated cleanroom environments, and validated scientific molding practices, manufacturers can deliver components that are critical for modern healthcare.
-
Polycarbonate is widely used in medical devices due to its impact resistance and optical clarity; this resource details its engineering properties. ↩
-
This FDA guide outlines the critical requirements for sterilization processes to ensure medical devices are free from viable microorganisms. ↩
-
ISO 13485 is the international standard for Quality Management Systems in the medical device industry, establishing the baseline for regulatory compliance. ↩